Health effects of Chlorine Dioxide and Chlorine
BackIn this paragraph, an overview is given about the impact if the disinfectant in the treated water and/or in the water outfall.
Hypochlorite and Chlorine are used indifferently since in the aqueous solutions, at the equilibrium and depending on the pH, both are present in different concentrations.
Numerous studies have been conducted to evaluate the toxicity of chlorine dioxide and of its inorganic and organic by-products.
The oxidant action of chlorine dioxide, as was seen in the sections on the reactivity of CIO- ends with the formation of chlorites, chlorides and small quantities of chlorates.
The formation of chlorites is equal to about 60-70 % of the consumed chlorine dioxide or, to say, to 0.6 - 0.7 mg of CIO2 - per mg of CIO2 used up.
In the table below, characteristics of some disinfectants are reported.
The indicated index decreases from 1 to 4 (1 is the max, 4 is the min).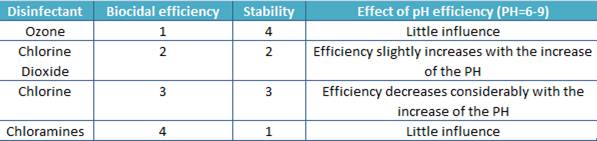
In the relevant applicative conditions of chlorine dioxide, in fact, the partial reduction of CIO2 into chlorite, that is the intermediate step of the reduction of chlorine dioxide into chloride, represents the prevalent reaction. The chlorates can be formed by the oxidation of hypochlorous acid (HCIO) on chlorite, resulting in turn from the reaction of CIO2with some organic substances, according to the following reaction:
HCIO + CIO2 - + OH- → CIO3-+ Cl-+ H2O
Furthermore, small quantities of hypochlorous acid, as a result of organic substances naturally present in water (humic acids and the likes), cause the formation of very limited quantities of Total Organic Halides (TOX). The formation of chlorites and chlorates can also take place through this breakdown of the CI02 in alkaline solutions.
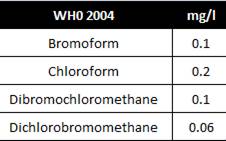
The presence of chlorates is linked to the efficiency of chlorine dioxide production and the potential photolysis after exposure to sunlight. Toxicological studies available today indicate that, of the dosages of chlorine dioxide, chlorites (CIO2 -) and chlorates (CIO -) used in water treatment do not present any risks to health. The results of clinical and biochemical studies, carried out in the United States, on the effects of chlorine dioxide ingested by means of regular consumption to water, indicate that the chlorite concentrations threshold beyond which there could be a certain effect on health is equal to 24 ppm for healthy individuals and 5 ppm for individuals affected with a deficiency of the G6PD enzyme (Glucose -6- Phosphate Dehydrogenase). Toxicological studies made on animals have shown that the concentration of chlorite at which haemolytic stress begins to manifest itself is 250 ppm.
Acceptable limits for chlorine dioxide and other compounds in drinking water are: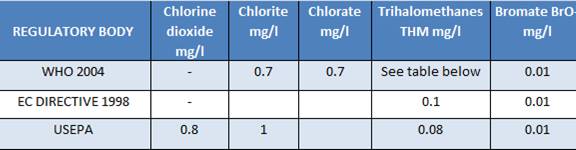
The sum of the ratio of the concentration of each to its respective guideline value should not exceed 1.
The limits established for the residual concentrations of chlorine dioxide, chlorites, and chlorates and other compounds in drinking water are shown in the Table above. It is worth noting the formation of halogenated organic compounds as by-products of the oxidation of soluble organic fractions (NOM: Natural Organic Matter), which include (up to 75 %) humic and fulvic acids, present in the water. Since it is practically impossible to identify systematically and completely all of these halogenated compounds, methods and definitions have been adopted conventionally to determine their overall content. These are the so-called “a-specific” parameters usually indicated as OX (Organic Halides) which are capable of evaluating different fractions of organic chlorinated products, in accordance with analytical techniques and the adopted experimental conditions. Table 9 gives the acronyms most commonly used to identify the above mentioned fractions.
At the present state of the art, trihalomethanes (THM) constitute an effective indicator of the total content of halogenated organic compounds and are commonly used in evaluating the disinfection process as regards to the formation of these kind of by-products.
Evaluation of the THM, as well, is logically justified, because they represent the fraction which is potentially most toxic to humans’ health.
THM refers to the following four products:
• Chloroform (CHCI3);
• Dichlorobromomethane (CHCI2Br);
• Dibromochloromethane (CHBr2CI);
• Bromoform (CHBr3).
These chemicals have low boiling points, may already be present in water subject to the purifying process polluted by industrial activities. Except in cases of specific contamination, their concentration is normally very limited, at the level of the “detection limit” of survey methods presently available.
THM constitute the “lightest” fraction of the family of chlorinated organics to which they belong. These molecules have been partially identified, but some are still unknown because of the analytical difficulty of their determination.
In recent years, with the refinement of various methods of specific analysis, it has been possible to assess quantitatively not only THM, but other subgroups of the same family, such as haloigenacetic acids, halogenacetonitriles, trichlorophenol and other aromatic and aliphatic hydrocarbons.
A comparison of the effect on THM formation during disinfection between chlorine dioxide and chlorine is given below: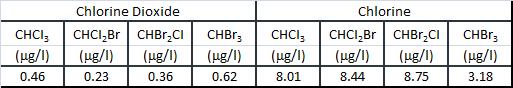
As a matter of fact, chlorine treatment promotes THM formation, while THM formation is negligible for chlorine dioxide.
Chlorine dioxide shows a very slight chlorinating action, since its degradation contributes only minimally to the formation of hypochlorous acid which, in addition to oxidation also causes addition and substitution reactions (and therefore, chlorination reactions).
Furthermore, in comparison with chlorine, it can be said that chlorine dioxide does not produce THM, as it may be when comparing the formation of chloroform (CHCI3) in the treatments of water containing around 5 mg/l of humic acid with chlorine and chlorine dioxide. The comparison between the chlorinating action of chlorine and that of chlorine dioxide is also pointed out by a study which describes the formation of bromo-methanes, in addition to that of chloroform. The formation of bromo-methanes is linked to the oxidation of bromine into hypobromous acid, which reacts with the humic substances. Chlorine dioxide, which does not react with bromine, does not cause the formation of bromo-methanes, except after photolysis and, thus, after exposure to light. The quantities of TOX and AOX measured in water treated with chlorine dioxide are minimal, in percentages varying from 1 % to 25 % as compared to that produced by chlorine.
It may be due in part to the presence of chlorine residue in the ClO2 produced from chlorite and chlorine, and, in part, according to Rice, to the direct action of chlorine dioxide, with the formation at pH = 3 and 7.8 of 4 classes of oxidation by-products: benzenepolycarboxylic acid, dibasic aliphatic acids, carboxyphenylglucosilic acids and monobasic aliphatic acids. It must be pointed out that the by-products of chlorine dioxide oxidation possess neither any acute or chronic toxicity, nor any mutagenic or carcinogenic properties. If utilized in pre-oxidation, chlorine dioxide considerably reduces the potential formation of THM and TOX. Studies addressing the reduction of potential formation of THM by means of ClO2 have shown that it acts on the precursors making them not reactive or unavailable for the formation of halomethanes.